Detoxicology
Cleaning up the world, one person at a time
Are we reaching crisis point for human fertility?
Fertility and Toxicity
Dr F. Ray MSc Applied Toxicology, MSc Chiropractic
The Problem.
We are becoming less fertile.
Women are having less children than ever before, 15-25% of couples in western countries fail to conceive after 12 months of unprotected intercourse. Impaired fecundity may affect twice that number[1]. Many women in their late thirties and early 40’s are finding it unexpectedly difficult to concieve. Many couples seeking the help of reproductive medicine are suddenly faced with issues such as poor sperm count/ quality, poor ovarian reserve, poor egg quality and other issues that indicate the ovaries and testes are old before their time.
Sperm counts are declining at ever faster rates. The average sperm count has dropped from 113 million to 66 million between 1934 and 1991[2]. That is a drop of almost ½. From 1980 to 2015 we have a 57% drop[3]. There is evidence the rate of drop in sperm count is increasing. Sperm counts are also seen to decrease by more recent year of birth, suggesting in-utero factors affecting fetal serloti cell function and multiplication, linked to lower sperm counts, higher risks of testicular cancer, hypospadias, and undescended testicles[4]. Humans have been somewhat overblessed on sperm count, so a count of 50m or above had a fairly similar effect. Many men have levels below this threshold. We are now rapidly approaching the 40m level as an average sperm count. At this level there is a decreased monthly probability of conception[5]. Why?
This overview from Sengupta et al 2015 list possible factors for male infertility[6]
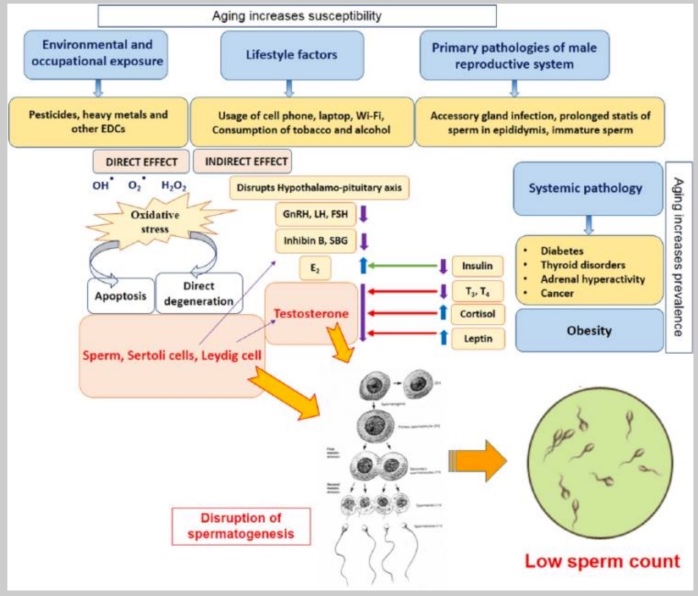
Micronutrient depletion
For micronutrients there are multiple issues, from soil demineralisation to increased processing of foods leading to the stripping out of vital nutrients, to increased demand for certain micronutrients due to environmental pollution.
Soil demineralisation
In the last 60 years a huge revolution has taken place in the way we grow our food. The use of modern farming practices, fertilizers and pesticides has increased the yield from each acre of land. Vegetables and fruit are now much bigger than they used to be. This however has produced a new problem. Bigger is not necessarily better – as fruit and vegetables get larger, their vitamin and mineral levels decline, as does the taste in most instances. Effectively the nutrient levels in foods have been diluted in the chase for a higher yield[7]. Milk from high production cows has less calcium than milk from dairies 20 years ago. Wheat has shown similar problems, with lower levels of iron, zinc and calcium, meaning you now have to eat twice as much to get the same nutritional value compared to older ways of growing the grain[8]
Another issue affecting the vitamin and mineral level is the wide scale depletion of the topsoil and soil mineral levels. The Rio earth summit in 1992 raised concern over the disappearance of many trace minerals from the soil. Over the last 100 years soil mineral levels have declined by 76% in Asia, 85% in the USA and 72% in Europe. Much of the blame can be placed with artificial fertilizers, that only replace four or five minerals compared with the 92 or so that plants remove from the soil. Chemical methods of farming have also affected the soil bacteria who make minerals available to plants in a way they can absorb. Pesticides and herbicides can also reduce the level of mineral uptake by plants.
The effect of this is that our fruit and veg no longer provide us with the vitamin and mineral levels we need for health. In a series of 5 reports complied for the medical research council, the Ministry of Agriculture, Fisheries and Food in the UK, have traced the mineral content of foods from 1940-1991. Over that time they have noted average losses in vegetables of[9]:
- 49% of their sodium content
- 16% of their potassium
- 24% of their magnesium content
- 46% of their calcium content
- 27% of their iron content
- 76% of their copper content
- The exception – a 9% rise in phosphorous (due to use of nitrogen, phosphorous potassium fertilizer)
It must be noted that cooking times for the vegetables tested has decreased in the testing period. E.g. broccoli is now analyzed having been cooked for 15 minutes, not the 45 minutes it was cooked for in 1940. Losses in cooking water would have been higher in 1940 than in 1991, so the nutrient loss over the years could actually be even higher. Demineralization of our food is speeding up too – the data from 1978-1991 shows much of the loss has happened in the last 13 years. Between 1978-1991, vegetables had an average:
- Loss of 39% of their sodium content
- Loss of 16% of their potassium content
- Loss of 14% of their phosphorous content
- Loss of 33% of their magnesium content
- Loss of 40% of their calcium content
- Increase of 6% iron content
- Loss of 72% copper content
- Loss of 59% zinc content
Fruits have shown similar but less steep declines in nutrient value. series of reports was the nutrient value of meat over the 51-year period. Average mineral losses included a 41% decrease in magnesium and a 54% decrease in iron. Trace minerals were most likely severely depleted if the 24% loss in copper is any indication. Dairy produce also shows a steep drop in magnesium. This is of particular concern as there is considerable evidence that bone mineral density is very dependent on magnesium levels, and milk is already low on magnesium compared to calcium. . There is a glimmer of hope however, organically produced food, particularly locally produced fruit and vegetables, have been shown to have higher vitamin and mineral levels than non organic food. Organic farmers have also been found to have a much higher sperm count
Zinc – vital in many different aspects of male fertility and sperm quality. Low seminal zinc levels are correlated with sperm quality and quantity[10],[11]. Female fertility also requires zinc for correct sexual development, ovulation and normal menstrual cycles. Zinc is also needed to counteract reactive oxygen species (ROS) formation, which can affect oocyte maturation, ovulation, luteolysis and follicle atresia[12]. Women undergoing IVF treatment were significantly more likely to have low follicular zinc levels than controls. Micronutrient supplementation in general, including zinc can significantly improve embryo quality in older women undergoing IVF or ICSI, with a significant increase in fertilization rate (66.7%)compared to folic acid supplementation only( 42.9%) . Those achieving at least one good quality embryo in an IVF cycle was 58% of the micronutrient supplementation group vs 36% of the folic acid supplementation only group[13].
Currently 11.7% of the US population are classed as having inadequate zinc intake[14]. The number below the level of optimum zinc intake will be far higher. In the UK the average consumption of zinc is 9.7mg, rather than the RDA of 15mg. Zinc levels in vegetables have dropped by 59% between 1970 and 1991[15]
Endochrine disruption due to phthalates such as DEHP is worsened in those with lower zinc levels and the two issues together magnify the disruption to maternal and foetal steroid hormone levels in pregnancy, leading to effects such as increased risk of pre-term birth, reproductive tract abnormalities, reduced sperm counts in the offspring and increased risks of cancer[16]. DEHP leads to accumulation of zinc in the liver thus lowering available levels for other uses.
Selenium – low selenium levels are found in those with low sperm count. Low glutathione levels occur as a result – selenium is needed for glutathione recycling.
Folic acid – vital for oocyte quality, maturation, fertilization and implantation as well as for neural tube development. Supplementation is associated with a shorter time to pregnancy, less risk of miscarriage, and less episodes of anovulation in healthy young women. Doses well above those recommended for avoiding neural tube defects were most advantageous[17]. Regulation of homocysteine levels in general appears to aid fertility.
B12. Insufficient levels of B12 have been found in more than ½ infertile women[18].
Nutrients lost through food processing.
Chromium – This mineral is needed for the genesis of new cells.. Low levels are associated with reduced fertility and low sperm counts. Refined grains and sugars have reduced chromium levels and eating these foods increases our need for chromium.
Magnesium – There is an inverse relationship between endometriosis and magnesium intake[19]. Red blood cell concentration of magnesium in women with premenstrual syndrome was lower than in those who do not suffer[20]. Magnesium has important roles in glutathione metabolism (regulation of ROS levels) and mitochondrial function[21]. Many European population studies have found generally inadequate levels of magnesium intake, and lower intake levels for females than males. One hundred years ago normal intake was around 500mg/day. Now US and European populations are getting from175-400 mg per day[22]. Processed foods that have had the whole grains reduced to their white versions, thus removing the germ and bran, and modern fertilizers are thought to contribute to the reduced intake of magnesium. Inflammation, oxidative stress and degenerative disease are all linked to low magnesium levels.
Omega 3 essential fatty acids – trans fats are associated with lower female fertility, saturated and trans fats with lower semen quality, higher omega 3 levels with improved fertility for both sexes[23]. Processed food tends to be very low in omega 3 oils. Modern farming methods with animal feed becoming more processed, the level of omega 3 oils in the animal is lower than in traditional grass fed meat and dairy.
Obesity
BMI of over 30 is becoming all too common in the developed world. It is associated with reduced fertility and reduced success for fertility treatments.
Higher body weight is associated with decreased total sperm count, lower sperm mitochondrial potential, increased sperm DNA damage and increased risk of infertility[24]. There is also an impact on the next generation, with altered birth weight and increased risks for metabolic syndrome, hypertension, kidney disease, fatty liver and reduced fertility. The mechanism behind this appears to be increased oxidative stress in the sperm. Different studies have shown that reduced calorie diets, increased exercise and micronutrient supplementation can improve sperm quality.
Female obesity is associated with a high degree of anovulation, irregular menses, subfertility and increased risk of miscarriage[25]. When assisted reproduction techniques are used, obese women are less likely to achieve pregnancy, less, likely to have fertilized oocytes, more likely to have lower quality embryos and more likely to miscarry. There appears to be abnormal endometrial development and implantation also. Obesity is associated with abnormal ovarian milleu (environment), with increased endoplasmic reticulum stress, increased oocyte lipid content and impaired nuclear maturation. All this is reflective of lipotoxic mechanisms similar to those seen in diabetic heart disease.
Pesticides and Endochrine disrupting chemicals.
Human studies on female fertility found many chemicals affected different facets of the reproductive process[26]:
Nonylphenol is negatively associated with plasma LH (lutenising hormone) levels
Pesticides are generally associated with reduced levels of sex steroid hormone production and reduced fertility. Earlier menopause was associated with higher levels of some pesticides. Animal studies on organochloride pesticides show multiple negative impacts on the ovaries, including reduced ovarian weight, follicle growth and oocyte viability. Uterine structure and function can also be affected.
Dioxins inhibit enzymes and hormone receptors within the steroidogenesis pathway. TCDD exposure in adulthood is also linked to decreased fertility, time to pregnancy and endometriosis.
Phthalates– Those with higher phthalate levels exhibit more inflammation and oxidative stress[27]. Phthalates also induce oxidative stress in the ovaries and testis too. Women undergoing IVF who had higher levels of DHEP, a common phthalate, had significantly less eggs retrieved and were far less likely to get pregnant than those with lower levels. Higher phthalate levels are also associated with endometriosis.
PCB’s are associated with reduced IVF embryo development,as well as subfertility, endometriosis and uterine fibroids.
Parabens are associated with reduced antral follicle numbers and shortened menstrual cycles. They are also associated with decreased sex steroid hormone levels and decreased fecundity.
Bisphenol A– Women undergoing IVF who have higher levels of BPA were significantly less likely to conceive than those with lower levels. Lower number of normally matured oocytes, lower levels of fertilization and lower oocyte yield in IVF in those with higher BPA levels[28]. studies have shown a wide range of effects on reproductive hormone levels, however these results have been inconsistent[29]. It is regarded as a reproductive toxin that interferes with steroid hormone formation and can cross the placenta. BPA has neurotoxic effects and alters developmental processes such as gametogenesis and brain development[30]. BPA is also found in human and animal milks[31]. Bisphenol S, thought to be a safer alternative is showing association with higher BMI and reduced sperm parameters[32].
Solvents – occupational exposure to a mix of solvents has been associated with a higher risk of miscarriage and longer time to pregnancy[33].
Cigarette smoke
Overall, cigarette smoke is a potent reproductive toxin for both men and women. Epigenetic changes in the sperm of smokers confers risks to the health of the offspring[34]. Smoking is associated with accumulation of lead and cadmium in the seminal plasma, reduced sperm motility, lower sperm counts and increased abnormal morphology of the sperm. Increased damage to the DNA of sperm is also seen. Paternal smoking is associated with a higher risk of leukaemia in the offspring. Higher rates of childhood cancers and birth defects are also seen.
Female fertility is also strongly negatively impacted by smoking tobacco, with increased risk of adverse reproductive outcomes including placenta abnormalities, low birth weight babies, increased risk of pre-eclampsia and pre-term delivery.
E-cigarette use is also of concern. Mouse studies have found delayed implantation of embryos and reduced weight gain in the offspring[35]. In addition, e-cigarette exposure in-utero has caused metabolic, inflammatory, neurologic and pulmonary factors in the offspring in mouse studies.
Cigarette smoke is toxic to mitochondria[36] and will affect the fertility of both sexes via this mechanism.
Air pollution .
Ultrafine particle matter is associated with increased intracellular oxidative stress, depleted glutathione and mitochondrial damage[37]. Ultra fine particle exposure (less than 0.1micrometers) from aircraft fumes is associated with an increased risk of pre-term birth.
For women undergoing IVF, exposure to nitrogen dioxide and ozone was associated with a reduced birth rate[38]. Particulate matter of 10(PM10)was associated with increased miscarriage. In the general population, PM 2.5-10 was linked with reduced fecundability and sulphur dioxide, carbon monoxide and nitrogen dioxide with with increased risk of miscarriage and stillbirth.
PM 2.5 exposure is negatively associated with sperm quality. Possible mechanisms as to the effect of PM on fertility include interaction with cellular recepters, promotion of oxidative stress and inflammation and DNA methylation.
Wifi, Cellphones and microwave radiation exposure.
Our electromagnetic environment has undergone exponential change over the last hundred years. It is becoming very clear that there is a litany of unintended effects on human health.
Devices emitting radiofrequency EMF radiation such as cell phones, wifi, laptops and microwave ovens, collectively termed electrosmog has been linked to many issues within fertility.
Effects on male fertility are marked. Continuous use of cellphones is linked to reduced sperm motility, sperm concentration/count, poor viability and abnormal morphology[39]. The mechanism of damage appears to originate with increased ROS formed in the mitochondria. Wifi exposure from laptops decreases sperm count and increases DNA fragmentation[40]. Military radar operators were found to have lower ejaculate volume and lower sperm count than a similar military control group[41].
Female fertility is also impacted by EMF, with higher levels of reactive oxygen species formed due to electron leakage from the mitochondrial electron transport chains as the main mechanism of damage[42]. This leads to poor quality pre-implantation embryos. Eggs have approximately 100,000 mitochondria to fuel the initial cell division process, whereas sperm have about 1000.
Using a mobile phone for more than an hour in pregnancy was found to alter biochemical parameters of cord blood (tested after delivery of the baby).
In general EMF exposure reduces the anitoxidant protection within cells, including those of the ovary and testes. This is related to down regulation of transcriptional and protein levels of antioxidant genes (SOD1,SOD2, CAT and GPx1). EMF exposure becomes a profound mitochondrial toxin.
Toxic metals
Mercury
Mercury affects female and male fertility via mechanisms that are not well understood[43]. Increased mercury levels in urine, hair and blood were associated with reduced fertility, increased damage to the DNA, motility and morphology of sperm. In women, increased incidence of menstrual and hormonal disorders, as well as increased incidences of adverse reproductive outcomes were seen with higher mercury levels. Reduced fertility has been seen in dental health care workers who were occupationally exposed to mercury[44]. Paternal occupational exposure to mercury leading to urinary mercury levels of 50micrograms/litre was found to double the rate of miscarriage in one study[45]
Heavy metals, of which mercury is one, are inversely associated with blood concentrations of pituitary gland hormones and LH levels[46]. They are also associated with decreased mature oocytes and oocyte yield following ovarian stimulation. Uterine fibroids are also associated with heavy metal body levels. Rodent studies have also found decreased uterine size, height of epithelial cells and endometrial glands, and decreased myometrium thickness.
Mercury (Hg) in hair is also negatively correlated with oocyte yields.
Maternal and cord blood levels have been significantly associated with higher risk of pre-term birth in some studies, whereas others found no significance.
Lead (Pb).
Lead also has many dofferent effects on fertility. It decreases the fluidity of the pituitary membrane in rats – this can affect hormone secretion and receptor binding[47]. Women undergoing IVF in Taranto, Italy, an area known for industrial heavy metal contamination, have higher follicular fluid levels of heavy metals (Cr and Pb )and reduced numbers of mature oocytes compared to women outside this region[48]. Urinary levels of Pb and Hg are associated with increased incidence of fibroids[49]. Paternal exposure to lead and mercury was linked with a higher chance of miscarriage[50].
Higher blood levels of lead are associated with unexplained infertility[51]. Higher blood and serum levels of Pb have also been found in women with pre-eclampsia and also has been seen to increase the odds of a spontaneous abortion, though studies vary on this finding.
Cadmium
Male blood lead levels and female blood cadmium levels (from environmental exposure) were both found to increase time to pregnancy[52].
Arsenic
Exposure through drinking water (4 mcg/ml and 0.4ppm) lowered LH and FSH levels in rats[53]. Other rodent studies have found decreased uterine size,myometrium thickness, fewer invaginations of the uterine lumen, fewer endometrial glands and reduced expression of vascular endothelial growth factor (VEGF) which is an oestrogen responsive gene in the endometrium.
Arsenic exposure with higher levels of urinary, hair and blood As has been linked to increased odds of pre-term birth, low birth weight and small for gestational age[54].
Cadmium
Tobacco smoke is known to contain high levels of cadmium[55]. Male fertility is adversely affected by cadmium exposure, with reduced spermatogenesis, reduced sperm quality and reduced hormone synthesis and release. Female fertility is affected with impaired hormonal balance and effects on the menstrual cycle. There is reduced pregnancy rates even at lower doses[56].
Overall toxic metal exposure was negatively linked to adverse pregnancy outcomes, fecundity and ovarian follicular health[57].
Conclusion
Human fertility is being hit by a perfect storm of toxicity, poor nutrition, environmental pollution, electro-smog and social factors affecting all aspects of fertility. We have disruption to sexual development from every point – in-utero to gamete formation, hormone production and regulation. Males are less male, and females can be less female. Given the early toxic effects of many of the toxins listed, we have a ticking time bomb for future human fertility and reproduction. It is possible that in the long term we may be driving ourselves to a reproductive crisis.
References
[1] Gaskins, A. J., & Chavarro, J. E. (2018). Diet and fertility: a review. American journal of obstetrics and gynecology, 218(4), 379–389. https://doi.org/10.1016/j.ajog.2017.08.010
[2] Carlsen, E., Giwercman, A., Keiding, N., & Skakkebaek, N. E. (1992). Evidence for decreasing quality of semen during past 50 years. BMJ (Clinical research ed.), 305(6854), 609–613. https://doi.org/10.1136/bmj.305.6854.609
[3] Sengupta, P., Dutta, S., & Krajewska-Kulak, E. (2017). The Disappearing Sperms: Analysis of Reports Published Between 1980 and 2015. American journal of men’s health, 11(4), 1279–1304. https://doi.org/10.1177/1557988316643383
[4] Sharpe R. M., Skakkebæk N. E. (1993). Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet, 341, 1392-1395.
[5] Hagai Levine, Niels Jørgensen, Anderson Martino-Andrade, Jaime Mendiola, Dan Weksler-Derri, Irina Mindlis, Rachel Pinotti, Shanna H Swan, Temporal trends in sperm count: a systematic review and meta-regression analysis, Human Reproduction Update, Volume 23, Issue 6, November-December 2017, Pages 646–659, https://doi.org/10.1093/humupd/dmx022
[6] Sengupta, P., Dutta, S., & Krajewska-Kulak, E. (2017). The Disappearing Sperms: Analysis of Reports Published Between 1980 and 2015. American journal of men’s health, 11(4), 1279–1304. https://doi.org/10.1177/1557988316643383
[7] Davis, D.R., Epp, M.D., Riorden, H.D. Changes in USDA food composition data for 43 garden crops 1950-1999. Am Coll Nut. 23 (6) 669-682. 2004
[8] Davis, D.R., Epp, M.D., Riorden, H.D. Changes in USDA food composition data for 43 garden crops 1950-1999. Am Coll Nut. 23 (6) 669-682. 2004
[9] Davis, D.R., Epp, M.D., Riorden, H.D. Changes in USDA food composition data for 43 garden crops 1950-1999. Am Coll Nut. 23 (6) 669-682. 2004
[10] Fallah, A., Mohammad-Hasani, A., & Colagar, A. H. (2018). Zinc is an Essential Element for Male Fertility: A Review of Zn Roles in Men’s Health, Germination, Sperm Quality, and Fertilization. Journal of reproduction & infertility, 19(2), 69–81.
[11] Colagar, A. H., Marzony, E. T., & Chaichi, M. J. (2009). Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutrition research (New York, N.Y.), 29(2), 82–88. https://doi.org/10.1016/j.nutres.2008.11.007
[12] Ebisch, I. M., Thomas, C. M., Peters, W. H., Braat, D. D., & Steegers-Theunissen, R. P. (2007). The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Human reproduction update, 13(2), 163–174. https://doi.org/10.1093/humupd/dml054
[13] Schaefer, E., & Nock, D. (2019). The Impact of Preconceptional Multiple-Micronutrient Supplementation on Female Fertility. Clinical medicine insights. Women’s health, 12, 1179562X19843868. https://doi.org/10.1177/1179562X19843868
[14] https://lpi.oregonstate.edu/mic/micronutrient-inadequacies/overview
[15] McCance, R.A., Widdowson, E.M. A study on the mineral depletion of the foods available to us as a nation over the period 1940-1991. MAFF . Compiled by D.E. Thomas http://www.organicgarden.org.uk/files/min_dep_report.pdf
[16] Nuttall, J. R., Kucera, H. R., Supasai, S., Gaikwad, N. W., & Oteiza, P. I. (2017). Combined Effects of Gestational Phthalate Exposure and Zinc Deficiency on Steroid Metabolism and Growth. Toxicological sciences : an official journal of the Society of Toxicology, 156(2), 469–479. https://doi.org/10.1093/toxsci/kfx008
[17] Gaskins, A. J., & Chavarro, J. E. (2018). Diet and fertility: a review. American journal of obstetrics and gynecology, 218(4), 379–389. https://doi.org/10.1016/j.ajog.2017.08.010
[18] Schaefer, E., & Nock, D. (2019). The Impact of Preconceptional Multiple-Micronutrient Supplementation on Female Fertility. Clinical medicine insights. Women’s health, 12, 1179562X19843868. https://doi.org/10.1177/1179562X19843868
[19] Harris, H. R., Chavarro, J. E., Malspeis, S., Willett, W. C., & Missmer, S. A. (2013). Dairy-food, calcium, magnesium, and vitamin D intake and endometriosis: a prospective cohort study. American journal of epidemiology, 177(5), 420–430. https://doi.org/10.1093/aje/kws247
[20] Sherwood, R. A., Rocks, B. F., Stewart, A., & Saxton, R. S. (1986). Magnesium and the premenstrual syndrome. Annals of clinical biochemistry, 23 ( Pt 6), 667–670. https://doi.org/10.1177/000456328602300607
[21] Gröber, U., Schmidt, J., & Kisters, K. (2015). Magnesium in Prevention and Therapy. Nutrients, 7(9), 8199–8226. https://doi.org/10.3390/nu7095388
[22] Gröber, U., Schmidt, J., & Kisters, K. (2015). Magnesium in Prevention and Therapy. Nutrients, 7(9), 8199–8226. https://doi.org/10.3390/nu7095388
[23] Gaskins, A. J., & Chavarro, J. E. (2018). Diet and fertility: a review. American journal of obstetrics and gynecology, 218(4), 379–389. https://doi.org/10.1016/j.ajog.2017.08.010
[24] McPherson, N. O., Shehadeh, H., Fullston, T., Zander-Fox, D. L., & Lane, M. (2019). Dietary Micronutrient Supplementation for 12 Days in Obese Male Mice Restores Sperm Oxidative Stress. Nutrients, 11(9), 2196. https://doi.org/10.3390/nu11092196
[25] Jungheim, E. S., Travieso, J. L., & Hopeman, M. M. (2013). Weighing the impact of obesity on female reproductive function and fertility. Nutrition reviews, 71 Suppl 1(0 1), S3–S8. https://doi.org/10.1111/nure.12056
[26] Rattan, S., Zhou, C., Chiang, C., Mahalingam, S., Brehm, E., & Flaws, J. A. (2017). Exposure to endocrine disruptors during adulthood: consequences for female fertility. The Journal of endocrinology, 233(3), R109–R129.
[27] Fett, R. 2019. It Starts with the Egg. 2nd Edition. 59-71. Franklin Fox Publishing, New York.
[28] Ehrlich, S., Williams, P. L., Missmer, S. A., Flaws, J. A., Ye, X., Calafat, A. M., Petrozza, J. C., Wright, D., & Hauser, R. (2012). Urinary bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Human reproduction (Oxford, England), 27(12), 3583–3592. https://doi.org/10.1093/humrep/des328
[29] Mínguez-Alarcón, L., Hauser, R., & Gaskins, A. J. (2016). Effects of bisphenol A on male and couple reproductive health: a review. Fertility and sterility, 106(4), 864–870. https://doi.org/10.1016/j.fertnstert.2016.07.1118
[30] Santoro, A., Chianese, R., Troisi, J., Richards, S., Nori, S. L., Fasano, S., Guida, M., Plunk, E., Viggiano, A., Pierantoni, R., & Meccariello, R. (2019). Neuro-toxic and Reproductive Effects of BPA. Current neuropharmacology, 17(12), 1109–1132. https://doi.org/10.2174/1570159X17666190726112101
[31] Mercogliano, R., & Santonicola, S. (2018). Investigation on bisphenol A levels in human milk and dairy supply chain: A review. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 114, 98–107. https://doi.org/10.1016/j.fct.2018.02.021
[32] Ghayda, R. A., Williams, P. L., Chavarro, J. E., Ford, J. B., Souter, I., Calafat, A. M., Hauser, R., & Mínguez-Alarcón, L. (2019). Urinary bisphenol S concentrations: Potential predictors of and associations with semen quality parameters among men attending a fertility center. Environment international, 131, 105050. https://doi.org/10.1016/j.envint.2019.105050
[33] Attarchi, M. S., Ashouri, M., Labbafinejad, Y., & Mohammadi, S. (2012). Assessment of time to pregnancy and spontaneous abortion status following occupational exposure to organic solvents mixture. International archives of occupational and environmental health, 85(3), 295–303. https://doi.org/10.1007/s00420-011-0666-z
[34] Jenkins, T. G., James, E. R., Alonso, D. F., Hoidal, J. R., Murphy, P. J., Hotaling, J. M., Cairns, B. R., Carrell, D. T., & Aston, K. I. (2017). Cigarette smoking significantly alters sperm DNA methylation patterns. Andrology, 5(6), 1089–1099. https://doi.org/10.1111/andr.12416
[35] Wetendorf, M., Randall, L. T., Lemma, M. T., Hurr, S. H., Pawlak, J. B., Tarran, R., Doerschuk, C. M., & Caron, K. M. (2019). E-Cigarette Exposure Delays Implantation and Causes Reduced Weight Gain in Female Offspring Exposed In Utero. Journal of the Endocrine Society, 3(10), 1907–1916. https://doi.org/10.1210/js.2019-00216
[36] Fetterman, J. L., Sammy, M. J., & Ballinger, S. W. (2017). Mitochondrial toxicity of tobacco smoke and air pollution. Toxicology, 391, 18–33. https://doi.org/10.1016/j.tox.2017.08.002
[37] Li, N., Sioutas, C., Cho, A., Schmitz, D., Misra, C., Sempf, J., Wang, M., Oberley, T., Froines, J., & Nel, A. (2003). Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environmental health perspectives, 111(4), 455–460. https://doi.org/10.1289/ehp.6000
[38] Conforti, A., Mascia, M., Cioffi, G., De Angelis, C., Coppola, G., De Rosa, P., Pivonello, R., Alviggi, C., & De Placido, G. (2018). Air pollution and female fertility: a systematic review of literature. Reproductive biology and endocrinology : RB&E, 16(1), 117. https://doi.org/10.1186/s12958-018-0433-z
[39] Agarwal A, Deepinder F, Sharma RK, Ranga G, Li J. Effect of cell phone usage on semen analysis in men attending infertility clinic: an observational study. Fertil Steril. 2008;89:124–128. doi: 10.1016/j.fertnstert.2007.01.166.
[40] Avendano C, Mata A, Sarmiento CS, Doncel G. Use of laptop computers connected to internet through Wi-fi decreases human sperm motility and increases sperm DNA fragmentation. Fertil Steril. 2012;97:39–45. doi: 10.1016/j.fertnstert.2011.10.012.
[41] Weyandt T. B., Schrader S. M., Turner T. W., Simon S. D. (1996). Semen analysis of military personnel associated with military duty assignments. Reproductive Toxicology, 10, 521-528.
[42] Santini, S. J., Cordone, V., Falone, S., Mijit, M., Tatone, C., Amicarelli, F., & Di Emidio, G. (2018). Role of Mitochondria in the Oxidative Stress Induced by Electromagnetic Fields: Focus on Reproductive Systems. Oxidative medicine and cellular longevity, 2018, 5076271. https://doi.org/10.1155/2018/5076271
[43] Henriques, M. C., Loureiro, S., Fardilha, M., & Herdeiro, M. T. (2019). Exposure to mercury and human reproductive health: A systematic review. Reproductive toxicology (Elmsford, N.Y.), 85, 93–103.
[44] Colquitt PJ. The effect of occupational exposure to mercury vapour on the fertility of female dental assistants. Occupational and Environmental Medicine. 1995;52:214.
[45] Cordier, S., Deplan, F., Mandereau, L., & Hemon, D. (1991). Paternal exposure to mercury and spontaneous abortions. British journal of industrial medicine, 48(6), 375–381. https://doi.org/10.1136/oem.48.6.375
[46] Rattan, S., Zhou, C., Chiang, C., Mahalingam, S., Brehm, E., & Flaws, J. A. (2017). Exposure to endocrine disruptors during adulthood: consequences for female fertility. The Journal of endocrinology, 233(3), R109–R129. https://doi.org/10.1530/JOE-17-0023
[47] Pillai A, Laxmi Priya PN, Gupta S. Effects of combined exposure to lead and cadmium on pituitary membrane of female rats. Arch Toxicol. 2002;76:671–675.
[48] Cavallini A, Lippolis C, Vacca M, Nardelli C, Castegna A, Arnesano F, Carella N, Depalo R. The Effects of Chronic Lifelong Activation of the AHR Pathway by Industrial Chemical Pollutants on Female Human Reproduction. PLoS One. 2016;11:e0152181
[49] Johnstone EB, Louis GM, Parsons PJ, Steuerwald AJ, Palmer CD, Chen Z, Sun L, Hammoud AO, Dorais J, Peterson CM. Increased urinary cobalt and whole blood concentrations of cadmium and lead in women with uterine leiomyomata: Findings from the ENDO Study. Reprod Toxicol. 2014;49:27–32.
[50] Anttila, A., & Sallmén, M. (1995). Effects of parental occupational exposure to lead and other metals on spontaneous abortion. Journal of occupational and environmental medicine, 37(8), 915–921. https://doi.org/10.1097/00043764-199508000-00005
[51] Rahman SN, Fatima P, Chowdhury AQ, Rahman MW. Blood level of lead in women with unexplained infertility. Mymensingh Med J. 2013;22:508–512.
[52] Buck Louis, G. M., Sundaram, R., Schisterman, E. F., Sweeney, A. M., Lynch, C. D., Gore-Langton, R. E., Chen, Z., Kim, S., Caldwell, K. L., & Barr, D. B. (2012). Heavy metals and couple fecundity, the LIFE Study. Chemosphere, 87(11), 1201–1207. https://doi.org/10.1016/j.chemosphere.2012.01.017
[53] Chattopadhyay S, Ghosh D. The involvement of hypophyseal-gonadal and hypophyseal-adrenal axes in arsenic-mediated ovarian and uterine toxicity: modulation by hCG. J Biochem Mol Toxicol. 2010;24:29–41.
[54] Rattan, S., Zhou, C., Chiang, C., Mahalingam, S., Brehm, E., & Flaws, J. A. (2017). Exposure to endocrine disruptors during adulthood: consequences for female fertility. The Journal of endocrinology, 233(3), R109–R129. https://doi.org/10.1530/JOE-17-0023
[55] Henson, M. C., & Chedrese, P. J. (2004). Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Experimental biology and medicine (Maywood, N.J.), 229(5), 383–392. https://doi.org/10.1177/153537020422900506
[56] Kumar, S., & Sharma, A. (2019). Cadmium toxicity: effects on human reproduction and fertility. Reviews on environmental health, 34(4), 327–338. https://doi.org/10.1515/reveh-2019-0016
[57] Rattan, S., Zhou, C., Chiang, C., Mahalingam, S., Brehm, E., & Flaws, J. A. (2017). Exposure to endocrine disruptors during adulthood: consequences for female fertility. The Journal of endocrinology, 233(3), R109–R129. https://doi.org/10.1530/JOE-17-0023